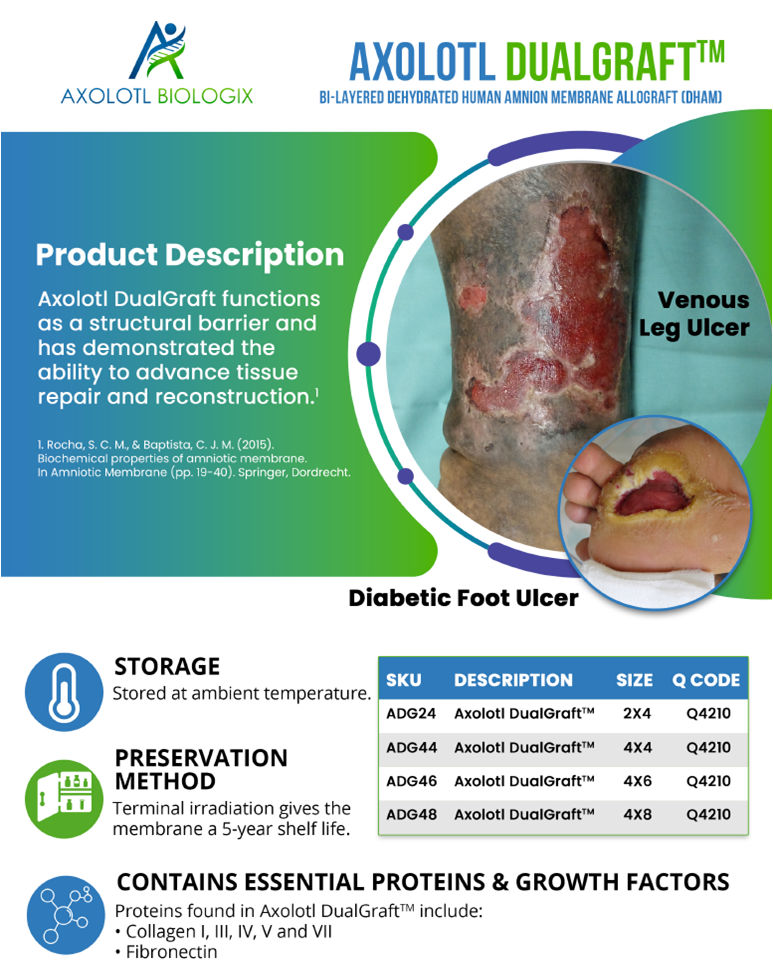
Latest Wound Care Allografts

Dual layer amniotic membrane.
carePATCH™ is a placental tissue allograft that may be used as a protective barrier in wound care applications. The natural properties of amniotic tissue provide mechanical protection and growth factors to support the management of acute and chronic wounds.
This is homologous use because serving as a natural scaffold as well as covering and offering protection from the surrounding environment are the inherent functions of amniotic membrane.
Proprietary processing of the tissue ensures that the natural structure and relevant characteristics are preserved.
Dehydrated, packaged, and terminally sterilized with a 2-year shelf life. Stored at ambient temperature.
HCPCS: Q4236
| SKU | DESCRIPTION | SIZE | UNITS | UPC |
|---|---|---|---|---|
| CPT022S | carePATCH™ Amniotic Membrane | 2x2cm | 4 | 382567000014 |
| CPT023S | carePATCH™ Amniotic Membrane | 2x3cm | 6 | 382567000090 |
| CPT024S | carePATCH™ Amniotic Membrane | 2x4cm | 8 | 382567000021 |
| CPT044S | carePATCH™ Amniotic Membrane | 4x4cm | 16 | 382567000038 |
| CPT046S | carePATCH™ Amniotic Membrane | 4x6cm | 24 | 382567000045 |
| CPT048S | carePATCH™ Amniotic Membrane | 4x8cm | 32 | 382567000059 |
| CPT312S | carePATCH™ Amniotic Membrane | 12mm | 1 | 382567000106 |
| CPT316S | carePATCH™ Amniotic Membrane | 16mm | 2 | 382567000113 |

 Dual layer amniotic membrane.
Dual layer amniotic membrane.
- barrera™ is a placental tissue allograft that may be used as a protective barrier in wound care applications. The natural properties of amniotic tissue provide mechanical protection and growth factors to support the management of acute and chronic wounds.
- This is homologous use because serving as a natural scaffold as well as covering and offering protection from the surrounding environment are the inherent functions of amniotic membrane.
- Proprietary processing of the tissue ensures that the natural structure and relevant characteristics are preserved.
- Dehydrated, packaged, and terminally sterilized with a 2-year shelf life. Stored at ambient temperature.
- HCPCS: Q4281
| SKU | DESCRIPTION | SIZE | UNITS | UPC |
|---|---|---|---|---|
| BRD-022 | Barrera™ Dual Layer Amniotic Membrane | 2x2cm | 4 | 382567000137 |
| BRD-023 | Barrera™ Dual Layer Amniotic Membrane | 2x3cm | 6 | 382567000144 |
| BRD-024 | Barrera™ Dual Layer Amniotic Membrane | 2x4cm | 8 | 382567000151 |
| BRD-044 | Barrera™ Dual Layer Amniotic Membrane | 4x4cm | 16 | 382567000168 |
| BRD-046 | Barrera™ Dual Layer Amniotic Membrane | 4x6cm | 24 | 382567000175 |
| BRD-048 | Barrera™ Dual Layer Amniotic Membrane | 4x8cm | 32 | 382567000182 |
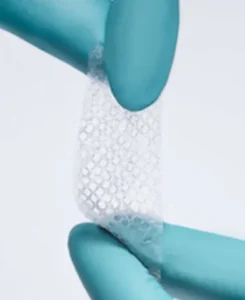
Durable Soft Tissue Covering Celera™ Patch is made up of amnion that has been processed to cleanse and decellularize the tissue while preserving the essential structural proteins and growth factors of the fetal basement membrane.
Natural Biological Scaffold Celera™ Patch is an Extracellular Matrix (ECM) isolated from placental tissue, one of nature’s most unique tissues that has remarkable strength and regenerative capacity.
Celera™ Patch is sterile and has a one to three year shelf life when stored according to the “Instructions for Use.” Donor tissue is obtained through a full-term birth consent program in partnership with FDA regulated and accredited recovery organizations.
Maternal screening includes:
- Hepatitis B core Anitgen (HBcAg)
- Hepatitis C antibodies (HCV Ab)
- Human Immunodeficiency Virus
- 1/O/2 antibodies (HIV-1/O/2 Ab)
- Human T-lymphotrophic virus I/II (HTLV-I/II)
Product Sizes
Dual Layer | Dual Membrane |
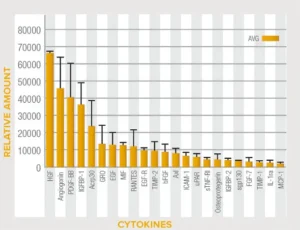
Celera™ Patch Cytokine Content*

Procenta® Description
- Acellular tissue allograft
- Contains loosely woven collagens
- Terminally Sterilized – SAL 10-6
- 4-Year shelf-life stored at ambient temperature
- Ready-to-Use: does not require re-hydration or rinsing of preservation media
- Requires no specific orientation
- Highly conformable
Intended Uses of Procenta®
- To serve as a cover
- To protect from the surrounding environment
- To retain fluid
Procenta® Application
- Open Procenta box and remove the product pouch
- Using aseptic technique, peel open the outer pouch containing sterile vial
- Open vial by the tear-off tab and remove the rubber stopper
- Remove product from the vial and apply to the treatment site using instrument such as a forceps
- Because Procenta is completely conformable, it requires no specific orientation on the treatment site
- Apply Procenta evenly over the surface of the wound
- Cover wound site with a non-adherent, impregnated dressing




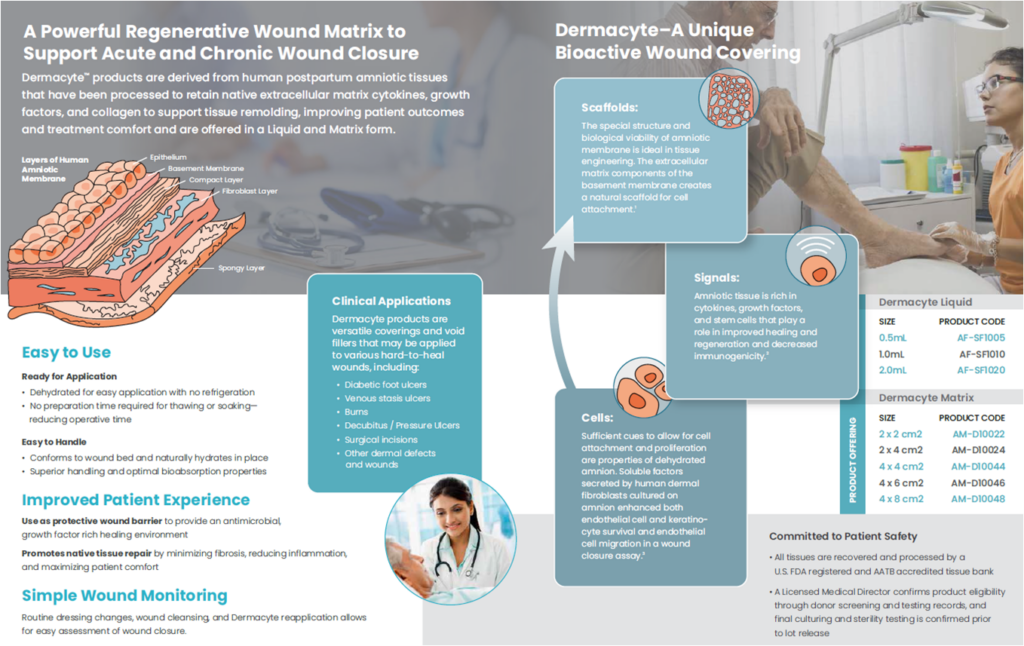
Dermacyte® Matrix
Dermacyte® Amniotic Wound Care Matrix (Dermacyte Matrix) is a human amniotic membrane allograft with a preserved epithelial basement membrane and compact fibroblast layer.
Dermacyte Matrix provides a protective extracellular matrix barrier for use in covering wounds and soft tissue. The product may be used as barrier to protect diabetic ulcers, venous ulcers, pressure ulcers, burn, and other dermal ulcerations that include wounds with exposed vital structures such as tendon, muscle, or bone.
Product SKU Identifier Size (L x W, centimeters)
AM-D10012 MT10012 1 x 2
AM-D10022 MT10022 2 x 2
AM-D10023 MT10023 2 x 3
AM-D10024 MT10024 2 x 4
AM-D10044 MT10044 4 x 4
AM-D10046 MT10046 4 x 6
AM-D10048 MT10048 4 x 8
AM-D10912 MT10912 9 X 12

XCELLERATE™ is an acellular, lyophilized amniotic membrane
- Two distinct membrane sides: an epithelial side and a stromal side.
- Aseptically processed to preserve extracellular matrix, growth factors and cytokines native to amniotic tissue*.
- XCELLERATE™ is a placental tissue allograft that may be used as a protective barrier in wound care applications. The natural properties of amniotic tissue provide mechanical protection and growth factors to support the management of acute and chronic wounds.
- This is homologous use because serving as a natural scaffold as well as covering and offering protection from the surrounding environment are the inherent functions of amniotic membrane.
- Proprietary processing of the tissue ensures that the natural structure and relevant characteristics are preserved.
- Dehydrated, packaged, and terminally sterilized with a 2-year shelf life. Stored at ambient temperature.
- HCPCS: Q4234
Ordering Information
| SKU | DESCRIPTION | SIZE | UNITS | UPC |
|---|---|---|---|---|
| XCELL-0202 | XCELLERATE ™ Lyophilized Amniotic Membrane | 2x2cm | 4 | 860004706533 |
| XCELL-0204 | XCELLERATE ™ Lyophilized Amniotic Membrane | 2x4cm | 8 | 860004706540 |
| XCELL-0404 | XCELLERATE ™ Lyophilized Amniotic Membrane | 4x4cm | 16 | 860004706557 |
| XCELL-0407 | XCELLERATE ™ Lyophilized Amniotic Membrane | 4x7cm | 28 | 860004706564 |


 Zenith™
Zenith™
Zenith Single-Layer Amniotic Graft is a sterile allograft designed for optimal wound covering and protection during the treatment of wounds.
- Provides a reliable protective wound covering backed by decades of science
- Dehydrated extracellular matrix acts as a scaffold supporting the native tissue
- Adheres easily to wounds including those with irregular surfaces
- Immune privileged and angiogenic
- 5-year shelf life at ambient temperature storage
- HCPCS: Q4253
Zenith Ordering Information
| PRODUCT NUMBER | SIZE | TOTAL UNITS (PER CM2) |
|---|---|---|
| ZNG-020202 | 2×2 | 4 |
| ZNG-020203 | 2×3 | 6 |
| ZNG-020404 | 4×4 | 16 |
| ZNG-020406 | 4×6 | 24 |
| ZNG-020408 | 4×8 | 32 |
| ZNG-021020 | 10×20 | 200 |
| ZNG-021520 | 15×20 | 300 |
Zenith Single-Layer Amniotic Graft is an amniotic membrane allograft derived from a prescreened mother with a planned C-section delivery. Zenith Single-Layer Amniotic Graft is manufactured in compliance with FDA regulations and AATB guidance. The membrane is minimally processed to preserve the native structure of the tissue, dehydrated, and terminally sterilized. Zenith Single-Layer Amniotic Graft is confirmed by the FDA Tissue Reference Group to meet the criteria for regulation solely under Section 361 of the PHS Act as defined in 21 CFR Part 1271.

Impax™
- Provides a protective wound covering
- Dehydrated extracellular matrix acts as a scaffold supporting the native tissue
- Adheres easily to wounds, including those with irregular surfaces
- Immune privileged and angiogenic
- 5-year shelf life at ambient temperature storage
Impax Ordering Information
| PRODUCT NUMBER | SIZE | TOTAL UNITS (PER CM2) |
|---|---|---|
| IMP-0202 | 2×2 | 4 |
| IMP-0203 | 2×3 | 6 |
| IMP-0404 | 4×4 | 16 |
| IMP-0406 | 4×6 | 24 |
| IMP-0408 | 4×8 | 32 |
| IMP-1020 | 10×20 | 200 |
| IMP-1520 | 15×20 | 300 |
Impax Dual-Layer Amniotic Graft is an amniotic membrane allograft derived from a prescreened mother with a planned C-section delivery. Impax Dual-Layer Amniotic Graft is manufactured in compliance with FDA regulations and AATB guidance. The membrane is minimally processed to preserve the native structure of the tissue, dehydrated, and terminally sterilized. Impax Dual-Layer Amniotic Graft is confirmed by the FDA Tissue Reference Group to meet the criteria for regulation solely under Section 361 of the PHS Act as defined in 21 CFR Part 1271.


- High purity type-I Collagen: Helicoll is a patented reconstituted bioactive collagen sheet, free of immunogenic proteins, lipids, and elastin. The native structure of the collagen is not altered or cross-linked which maintains its high bioactivity.
- Faster Healing: Collagen phosphorylation attracts cells, regenerates tissue, and stimulates blood capillaries/granulation within 4 to 5 days.
- Innovative Technology: Better than intact tissue-based membranes like an amnion, intestinal wall, urinary bladder, etc. which contain >15% elastin that is recently discovered to be carcinogenic.
- Easy Application: No washing needed prior to use. The overall clinical usage of Helicoll is simple and easy as it can be cut, sutured, or stapled.
- Pain Control: Effectively reduces pain.
- Various Sizes: Choose from standard or customized dimensions.
- Cost-Effective: Accelerated wound healing and tissue remodeling with minimal applications reduce the treatment cost by over 40%.
- Long Shelf Life: Remains clinically usable for 3 years when stored in room temperature conditions
Helicoll® Ordering Information Q4164
Catalog # | Description | Sheets per bx Box |
HC0.5dia | Helicoll, Biological Skin Substitute 0.5 in dia disc (1.27 cm dia disc = 1 sq cm) | 2 |
HC1.0dia | Helicoll, Biological Skin Substitute 1.0 in dia disc (2.54 cm dia disc = 5 sq cm) | 2 |
HC0.8×1.6 | Helicoll, Biological Skin Substitute 0.8in x 1.6in (2 cm x 4 cm = 8 sq cm) | 2 |
HC1.2×1.6 | Helicoll, Biological Skin Substitute 1.2 in x 1.6 in (3 cm x 4 cm = 12 sq cm) | 2 |
HC1.6×1.6 | Helicoll, Biological Skin Substitute 1.6 in x 1.6 in (4 cm x 4 cm = 16 sq cm) | 2 |
HC2x2 | Helicoll, Biological Skin Substitute 2 in x 2 in (5cm x 5cm=25 sq cm) | 5 |
HC2x4 | Helicoll, Biological Skin Substitute 2 in x 4 in (5cm x 10cm=50 sq cm) | 5 |

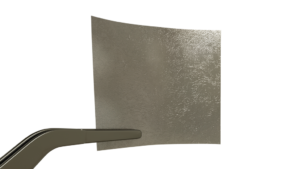
Full Thickness (Tripple Layer) Placental Membrane
- Multi-Layer graft processed using a proprietary processing technology to ensure all naturally occurring components of birth tissue remain intact through processing.
- Contains the Amnion, Chorion as well as the important Intermediate Layer (IL)/Spongy layer of the placenta.
- Amber color comes from advanced preservation of placental structures and layers.
- completeFT™ is a placental tissue allograft that may be used as a protective barrier in wound care applications. The natural properties of amniotic tissue provide mechanical protection and growth factors to support the management of acute and chronic wounds.
- This is homologous use because serving as a natural scaffold as well as covering and offering protection from the surrounding environment are the inherent functions of amniotic membrane.
- Dehydrated, packaged, and terminally sterilized with a 2-year shelf life. Stored at ambient temperature.
- HCPCS: Q4271F
| SKU | DESCRIPTION | SIZE | UNITS | UPC |
|---|---|---|---|---|
| EFT22 | Complete FT™ Placental Allograft Membrane | 2x2cm | 4 | 382567000137 |
| EFT24 | Complete FT™ Placental Allograft Membrane | 2x4cm | 8 | 382567000144 |
| EFT44 | Complete FT™ Placental Allograft Membrane | 4x4cm | 16 | 382567000151 |
| EFT36 | Complete FT™ Placental Allograft Membrane | 3x6cm | 18 | 382567000168 |
| EFT48 | Complete FT™ Placental Allograft Membrane | 4x8cm | 32 | 382567000175 |



Restorigin™ is a Dual Layer Amniotic Membrane
- Restorigin™ is a placental tissue allograft that may be used as a protective barrier in wound care applications. The natural properties of amniotic tissue provide mechanical protection and growth factors to support the management of acute and chronic wounds.
- This is homologous use because serving as a natural covering and offering protection from the surrounding environment are the inherent functions of amniotic membrane.
- Proprietary processing of the tissue ensures that the natural structure and relevant characteristics are preserved.
- Dehydrated, packaged, and terminally sterilized. Stored at ambient temperature with a 2-year shelf life.
- HCPCS: Q4191

| SKU | DESCRIPTION | SIZE | UNITS | UPC |
|---|---|---|---|---|
| RGN-AM-0312 | Restorigin™ Amnion Patch, Thin | 12mm Disc | 1 | 382567001738 |
| RGN-AM-0316 | Restorigin™ Amnion Patch, Thin | 16mm Disc | 2 | 382567001745 |
| RGN-AM-01515 | Restorigin™ Amnion Patch, Thin | 1.5×1.5cm | 1 | 382567002247 |
| RGN-AM-0202 | Restorigin™ Amnion Patch, Thin | 2x2cm | 4 | 382567000830 |
| RGN-AM-0203 | Restorigin™ Amnion Patch, Thin | 2x3cm | 6 | 382567000847 |
| RGN-AM-0204 | Restorigin™ Amnion Patch, Thin | 2x4cm | 8 | 382567000854 |
| RGN-AM-0404 | Restorigin™ Amnion Patch, Thin | 4x4cm | 16 | 382567000861 |
| RGN-AM-0406 | Restorigin™ Amnion Patch, Thin | 4x6cm | 24 | 382567000878 |
| RGN-AM-0505 | Restorigin™ Amnion Patch, Thin | 5x5cm | 25 | 382567001684 |
| RGN-AM-0408 | Restorigin™ Amnion Patch, Thin | 4x8cm | 32 | 382567000885 |



Recent Comments